Transferring Sponsorship of a Clinical Study: A lesson on how rare disease drug developers can work together for the good of an underserved rare disease community
On June 20th, 2019, wonderful news was announced on the Association for Creatine Deficiencies’ (ACD) website and ACD Facebook page that Lumos Pharma has transferred the sponsorship role of the Vigilan Study (Observational Study of Creatine Transporter Deficiency-CTD) to Ultragenyx Pharmaceutical Company.
“The ongoing natural history study, Vigilan, is crucial for the CTD patient and scientific communities”, commented Rick Hawkins, CEO, Lumos Pharma. “I am pleased that our Lumos team has been successful in transferring the Study to Ultragenyx. Rarely does one see this type of cooperative transition from one industry partner to another. It is a tribute to the respective teams of the NIH-TRND/NICHD, Lumos and Ultragenyx in helping facilitate the difficult transfer. The eventual benefit will most assuredly accrue to the CTD patients and families.”
When it was clear that Lumos could no longer continue sponsoring Vigilan due to the crushing discontinuation of our LUM-001 CTD program this past Spring, we were driven and determined to ensure that the Vigilan Study had the chance to continue. Fortunately, Ultragenyx, with interests in Creatine Transporter Deficiency, clearly understood our drive and sentiment that a natural history study like Vigilan is a critical and valuable resource for all, especially the patient community in their efforts to attract industry attention to invest and conduct respective research.
It has been our privilege to passionately work with the following stakeholders to make the Lumos-Ultragenyx Vigilan transfer a reality:
- the NIH, a prominent part of the Vigilan Study, including TRND and NICHD,
- the experienced and hard-working Vigilan sites,
- the superbly supportive patient community with their patient association ACD,
- and Ultragenyx, a drug developer who is deeply committed to improving the lives of patients with ultra-rare disorders.
In closing, rare disease drug developers share a common interest: working to help underserved individuals and families have therapeutic solutions for their devasting illnesses. Many rightfully say that they are putting the patient first and industry collaborations like these can contribute greatly to accomplishing that mission.
We are very pleased that this entire Lumos-Ultragenyx team has not just claimed this statement but has followed through with their actions.
With much hope to the future for the CTD community,
The Lumos Pharma Team
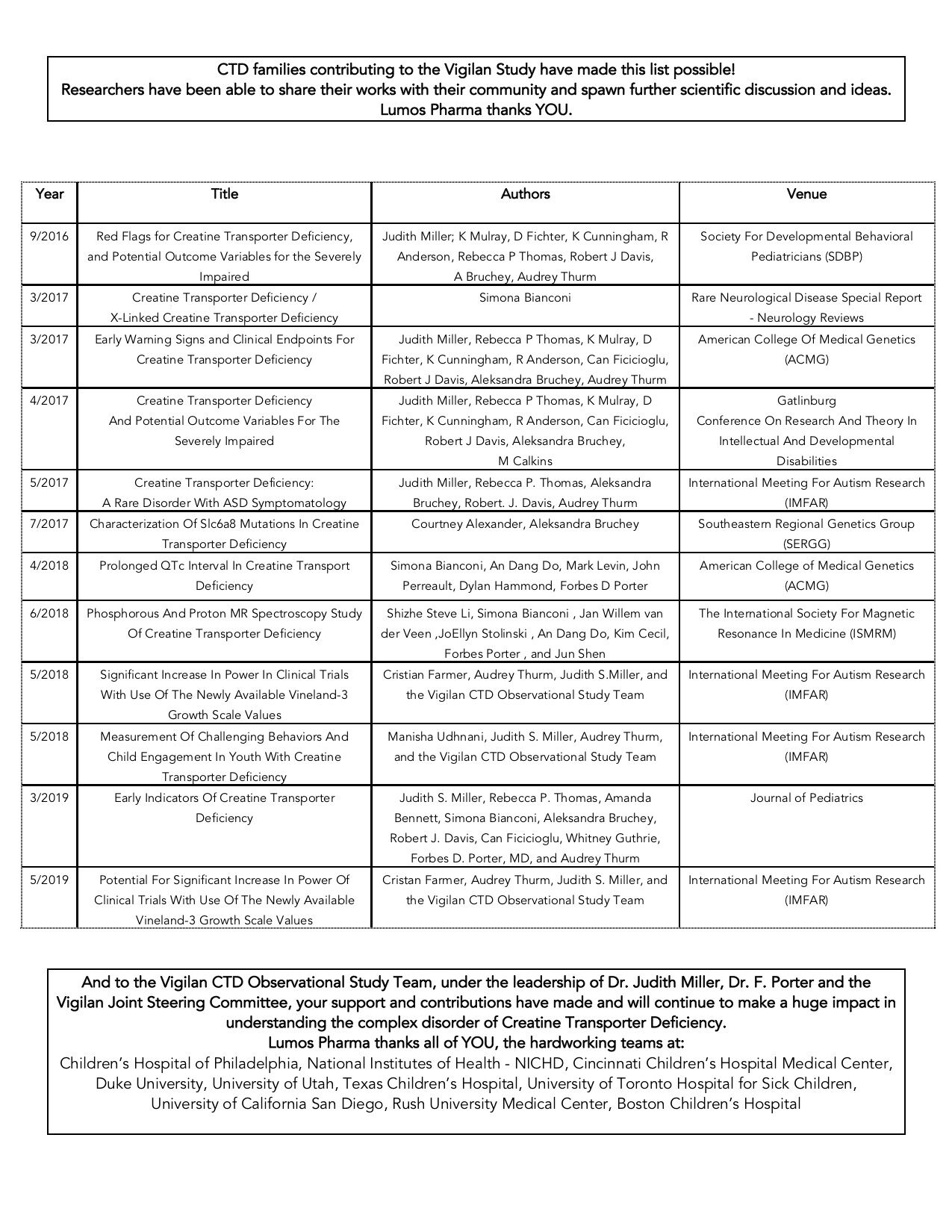
PS – We are extremely proud that even in the time we were at the helm of the Vigilan Study, the entire Vigilan team has already made such advances to disseminating important information on the study thus far through publications and scientific conference presentations. Take a look below.*
*Click the image to enlarge the table