OUR THERAPEUTIC DEVELOPMENT PROGRAMS FOR RARE DISEASES
Lumos Pharma is a clinical-stage biopharmaceutical company committed to identifying, developing and commercializing life-changing therapies for patients and families living with rare diseases
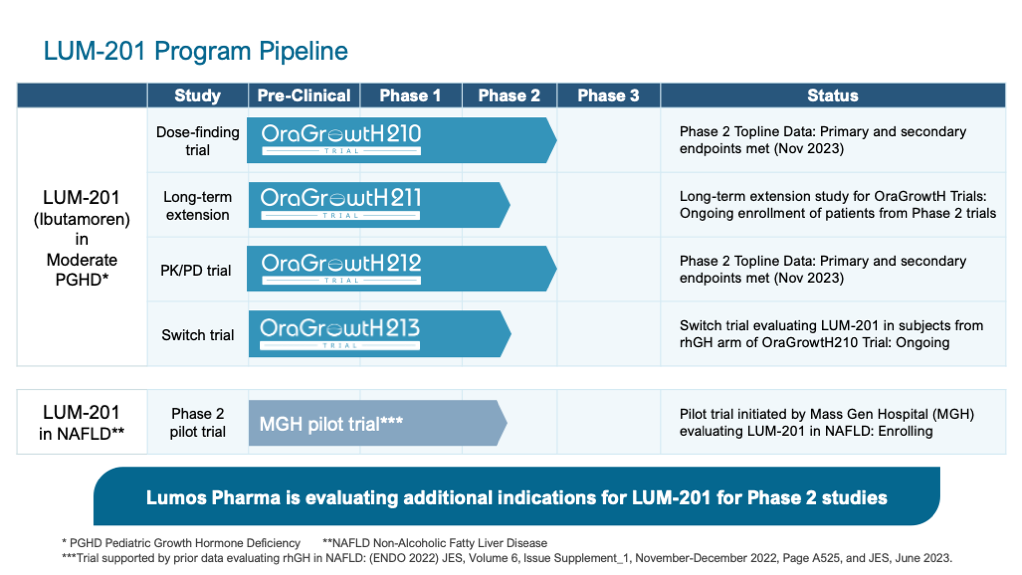
The Company’s pipeline features development programs in rare diseases associated with growth hormone deficiencies.
Lumos Pharma plans to expand its pipeline through strategic partnerships with those who share our mission of bringing innovative medicines to improve the lives of patients and families living with rare diseases.
LUM-201
- Also known as ibutamoren, LUM-201 is an orally administered small molecule that promotes the secretion (secretagogue) of Growth Hormone (GH) from the pituitary gland.1
- LUM-201 acts as an agonist of the GH Secretagogue Receptor to stimulate GH release.2
- LUM-201 has been observed to increase the amplitude of endogenous pulsatile GH secretion in humans, which mimics the natural pattern of GH secretion.3,4
- It has been studied in more than 1300 patients, both adult and pediatric, and was generally well tolerated.*
- Topline data from Phase 2 OraGrowtH210 and OraGrowtH212 Trials of LUM-201 in PGHD met all primary and secondary endpoints.
Important Information – *At the doses tested previously in the Merck Trials, LUM-201 was generally well-tolerated in children with the most commonly reported adverse events being digestive systems events, including appetite increase. Mild elevations in liver enzymes without accompanying changes in bilirubin were also reported.
References
- Patchett A.A. et al, Design and Biological Activities of L-163,191 (MK-0677): A Potent, Orally Active Growth Hormone Secretagogue, Proc Natl Acad Sci, 1995, 92:7001-7005
- Howard A.D. et al, A Receptor in Pituitary and Hypothalamus that Functions in Growth Hormone Release, Science, 1996, 273:974-977
- Nass R. et al, Effects of an Oral Ghrelin Mimetic on Body Composition and Clinical Outcomes in Healthy Older Adults, Ann Intern Med, 2008, 149:601-611
- Chapman I.M. et al, Oral Administration of Growth Hormone (GH) Releasing Peptide-Mimetic MK-677 Stimulates the GH/Insulin-Like Growth Factor-I Axis in Selected GH-Deficient Adults, J Clin Endocrinol Metab, 1997, 82(10):3455-3463