Presentations & Publications
LUM-201
ESPE 2024- Liverpool,UK – Late Breaking Oral Presentation
Oral Presentation:
”The Amount and Pattern Of Pulsatile GH Secretion Induced By The Oral Growth Hormone Secretagogue LUM-201 Is Related To Growth And IGF-1 Responses In Moderate Pediatric Growth Hormone Deficiency (PGHD)”
Nov 18, 2024
Presenting Author
Peter Clayton, MD, Univ. of Manchester, UK
View the EPSE 2024 Late Breaking Oral Presentation
ESPE 2024- Liverpool,UK – Oral Presentation
Oral Presentation:
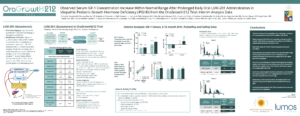
“Growth, IGF-1 and IGFBP-3 Responses to Oral LUM-201 in OraGrowtH210 and OraGrowtH212 Trials in Pediatric Growth Hormone Deficiency (PGHD) over 12 to 24 Months on Treatment”
Nov 17, 2024
Presenting Author
Elżbieta Petriczko, MD, Pomeranian Medical University in Szczecin, Poland
View the EPSE 2024 Late Breaking Oral Presentation
APPES 2024- New Delhi, India – Oral Presentation
Oral Presentation:
“Growth, IGF-1 and IGFBP-3 Responses to the Oral Growth Hormone (GH) Secretagogue, LUM-201, in Pediatric Growth Hormone Deficiency (PGHD) in the OraGrowtH210 Trial””
Oct 3, 2024
Presenting Author
Paul Hofman, MD, University of Auckland, New Zealand
View the APPES 2024 oral presentation
SLEP 2024 – Santiago, Chile, E-poster presentation
E-Poster Presentation:
“LUM-201 Restores Growth Hormone Secretion and Promotes Growth in Moderate Pediatric Growth Hormone Deficiency (PGHD): Phase 2 Topline Results from OraGrowtH210 and OraGrowtH212 Trials”
Sept 13, 2024
Presenting Author
Rossana Román, MD, Institute of Maternal and Child Research, University of Chile, Santiago, Chile
View Poster: SLEP 2024 Topline OraGrowtH210 and OraGrowtH212 Phase 2 Key Discoveries
ENDO 2024 – Boston, MA, USA – poster presentations
Poster Presentation (MON-111):
“Oral LUM-201 Restores Pulsatile Growth Hormone Secretion and Growth Response in Moderate Pediatric Growth Hormone Deficiency (PGHD): Key Discoveries from Phase 2 of OraGrowtH212 Trial”
June 3, 2024
Presenting Author
Fernando Cassorla, MD, University of Chile, Santiago, Chile
ENDO 2024 OraGrowtH212 Phase 2 Key Discoveries
Late Breaking Session: Poster Presentation (MON-704):
“Growth Response to Oral Growth Hormone Secretagogue LUM-201 in Children with Moderate GH Deficiency (GHD) is Dependent on the Pattern of Pulsatile GH Secretion Stimulated by LUM-201”
June 3, 2024
Presenting Author
Adam Stevens, PhD, University of Manchester, Manchester, UK
ENDO 2024 Late Breaking Poster Session
European Congress of Endocrinology (ECE) 2024- Stockholm,Sweden – poster presentation
Poster Presentation:
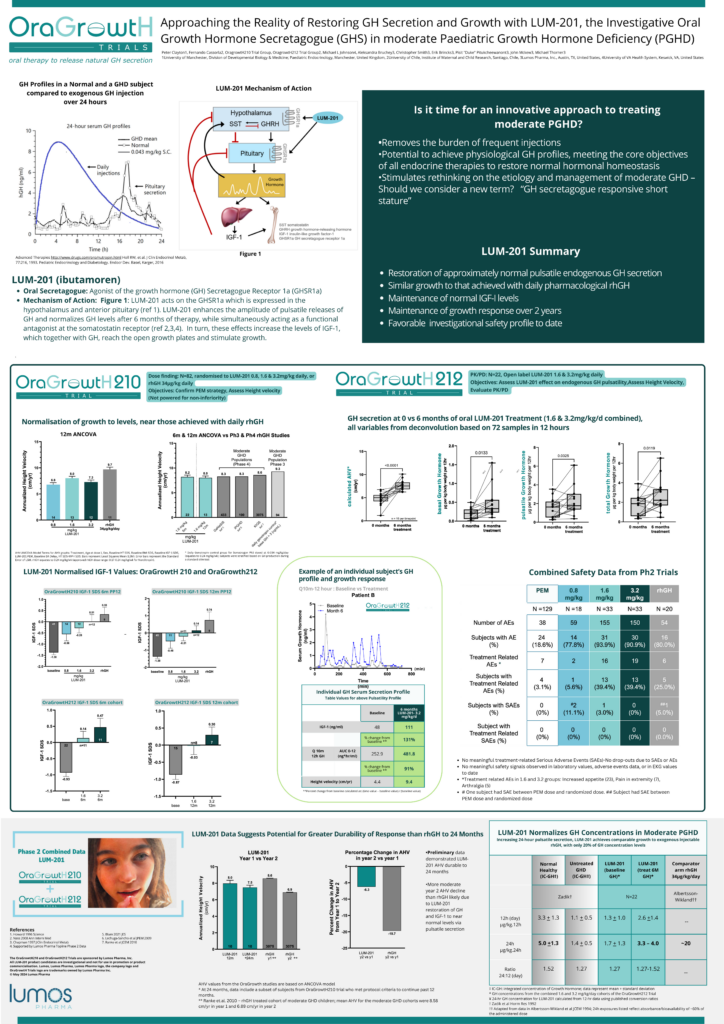
“Approaching the Reality of Restoring GH Secretion and Growth with LUM-201, the Investigative Oral Growth Hormone Secretagogue (GHS) in moderate Pediatric Growth Hormone Deficiency (PGHD)”
May 12, 2024
Presenting Author
Peter Clayton, MD, University of Manchester, Manchester, UK
Growth Research Society(GRS) 2024- Stockholm, Sweden – oral presentation
Oral Presentation:
“Approaching the Reality of Restoring GH Secretion and Growth with LUM-201, the Investigative Oral Growth Hormone Secretagogue (GHS) in moderate Pediatric Growth Hormone Deficiency (PGHD)”
May 10, 2024
Presenting Author
Peter Clayton, MD, University of Manchester, Manchester, UK
View the Growth Research Society 2024 Oral Presentation
Pediatric Endocrine Society PES 2024- Chicago, Illinois, USA – poster presentation
Poster Presentation:
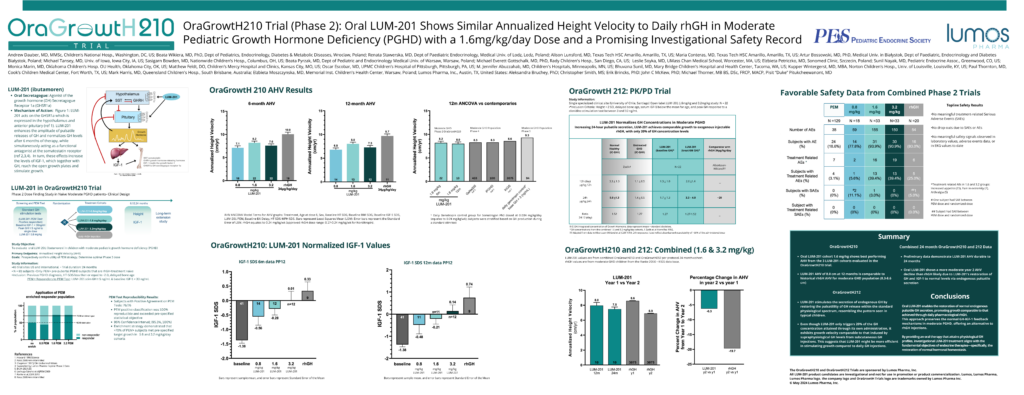
“OraGrowtH210 Trial (Phase 2): Oral LUM-201 Shows Similar Annualized Height Velocity to Daily rhGH in Moderate Pediatric Growth Hormone Deficiency (PGHD) with a 1.6mg/kg/day Dose and a Promising Investigational Safety Record”
May 3, 2024
Presenting Author
Andrew Dauber, MD,Children’s National Medical Center, Washington DC, USA
European Society of Pediatric Endocrinology (ESPE) 2023- The Hague, Netherlands – late breaking oral presentation
Late Breaking Oral Presentation:
“Deconvolution Analysis: Oral GH secretagogue (LUM-201) enhances growth in individuals with moderate Pediatric Growth Hormone Deficiency (PGHD) by enhancing endogenous GH secretion and increasing IGF-1”
September 23, 2023
Presenting Author
Fernando Cassorla, MD, University of Chile, Santiago, Chile
View the ESPE 2023 Late Breaking Oral Presentation
ENDO 2023- Chicago, Illinois, USA – two oral presentations
Oral Presentation:
“Response of Oral LUM-201 in OraGrowtH210 and OraGrowtH212 Trials in Idiopathic Pediatric Growth Hormone Deficiency (iPGHD): Combined Analysis Interim Analysis Data”
June 17, 2023
Presenting Author
Michael Tansey, MD, University of Iowa, Iowa City, Iowa USA
View the ENDO 2023 Presentation
Oral Presentation:
“Dose Responsiveness of LUM-201 as Measured by Acute GH Response and IGF-1 and Annualized Height Velocity (AHV) Measured at 6 Months in the Interim Analysis of the OraGrowtH212 Study in Idiopathic Pediatric Growth Hormone Deficiency (iPGHD)”
June 17, 2023
Presenting Author
Fernando Cassorla, MD, University of Chile, Santiago, Chile
View the ENDO 2023 Presentation
PES 2023- San Diego, California, USA – oral presentation and poster
Oral Presentation:
“Growth Response to LUM-201 in the OraGrowtH210 Trial in Idiopathic Pediatric Growth Hormone Deficiency (iPGHD): Interim Analysis Data (41 subjects)”
May 7, 2023
Presenting Author
Andrew Dauber, MD, Childrens National Medical Center, Washington DC, USA
View the PES 2023 Presentation
Poster:
“Observed Serum IGF-1 Concentration Increase Within Normal Range After Prolonged Daily Oral LUM-201 Administration in Idiopathic Pediatric Growth Hormone Deficiency from the OraGrowtH212 Trial: Interim Analysis Data”
May 6, 2023
IMPE 2023- Buenos Aires, Argentina – oral presentation and poster
Oral Presentation:
“Dose-dependent Increase in GH AUC0-12h with LUM- 201 in Idiopathic Pediatric GH Deficiency from the Interim Analysis Data of the OraGrowtH212 Trial”
March 5, 2023
Presenting Author
Fernando Cassorla, MD; University of Chile, Santiago, Chile
View the IMPE 2023 Presentation
Poster:
“LUM-201 in Idiopathic Pediatric Growth Hormone Deficiency (iPGHD) Interim Analysis on Baseline Demographics, Safety Profile and 6 month Annualized Height Velocity (AHV) from the OraGrowtH210 Trial”
March 5-7, 2023
Presenting Author
Alison Lunsford, MD, Texas Tech HSC, Amarillo, Texas, USA
ENDO 2021- virtually held meeting – poster
Poster: “LUM-201 Elicits Greater GH Response than Standard GH Secretagogues in Pediatric Growth Hormone Deficiency”
Presenting Author
George Bright, MD, Lumos Pharma, Austin, TX, USA.
PUBLICATIONS
LUM-201
Development of a Predictive Enrichment Marker for Oral GH Secretagogue LUM-201 in Children with Growth Hormone Deficiency
George M Bright, MD; Minh-Ha T Do, PhD; John C McKew, PhD; Werner F Blum, MD; Michael O Thorner, MB, BS, DSc
Journal of the Endocrine Society – bvab030
Published: 25 February 2021
Corroboration of Height Velocity Prediction Markers for rhGH with an Oral GH Secretagogue Treatment in Children with GHD
Werner F Blum, MD; George M Bright, MD; Minh-Ha T Do, PhD; John C McKew, PhD; Haiying Chen, MD, PhD; Michael O Thorner, MB, BS, DSc
Journal of the Endocrine Society – bvab029
Published: 25 February 2021
A GH Secretagogue Receptor Agonist (LUM-201) Elicits Greater GH Responses than Standard GH Secretagogues in Subjects of a Pediatric GH Deficiency Trial
George M Bright, MD; Michael O Thorner, MB, BS, DSc
Hormone Research in Pediatrics
DOI: 10.1159/000524244
Published: 30 March 2022